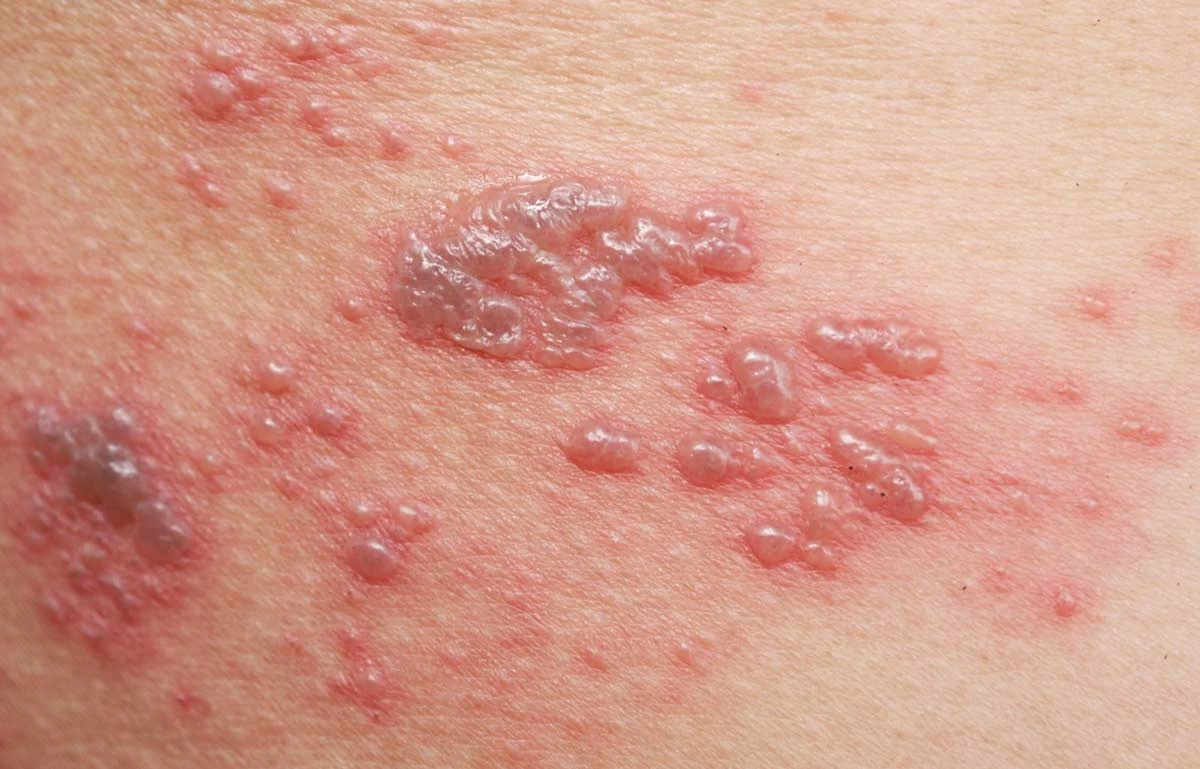
Shingles is also known as the “Belt of Fire”.
What is the purpose of this clinical research study?
The goal of this study is to develop a shingles vaccine with a better safety profile, production yields, and equivalent effectiveness to that of the leading vaccine on the market. Essentially, the vaccine being developed is for the prevention of shingles, also known as herpes zoster. Shingles is a viral disease by the varicella virus, the same virus that causes chickenpox.
Ages
Over 50
Study Topics
Vaccine
What is Shingles?
Shingles (Herpes Zoster) is a painful blistering skin rash caused by a reactivation of the varicella-zoster virus VZV. Shingles rash on the face near the eye can cause vision loss and other neurological problems. About 1 in 10 people with Shingles develop nerve pain, called postherpetic neuralgia (PHN) that does not go away for months or even years after the rash has resolved. In individuals with a weakened immune system, reactivation of VZV can be very serious and in some cases, life-threatening.
Study Eligibility Criteria
Ages 50 and over
Participants that have not already had a shingles vaccines
Participants that have not already had a shingles
Do You Not Meet the Criteria for This Study?
You could still participate in other studies. Contact us to be added to our database and you’ll be notified about our future studies that may be right for you.
Other Studies You May Be Interested In

Obesity

Norovirus (Food Poisoning)

Natural Weight Loss


